|
Pharmacies distributing Rapid Antigen Test (RAT) kits: Scroll down to Pharmacy Information - RAT Distribution to access the medSask COVID-19 Testing Assessment for Community Pharmacies form. Access to COVID-19 treatments has significantly changed since the federally funded Paxlovid™ program and other pandemic measures have ended. This webpage offers key updates and resources for healthcare providers to support safe and effective prescribing of COVID-19 antivirals, particularly Paxlovid™. To find a pharmacy dispensing Paxlovid™ in Saskatchewan, see this interactive map. Paxlovid™ Prescription for LAEAPaxlovid™ Prescription for LAEA
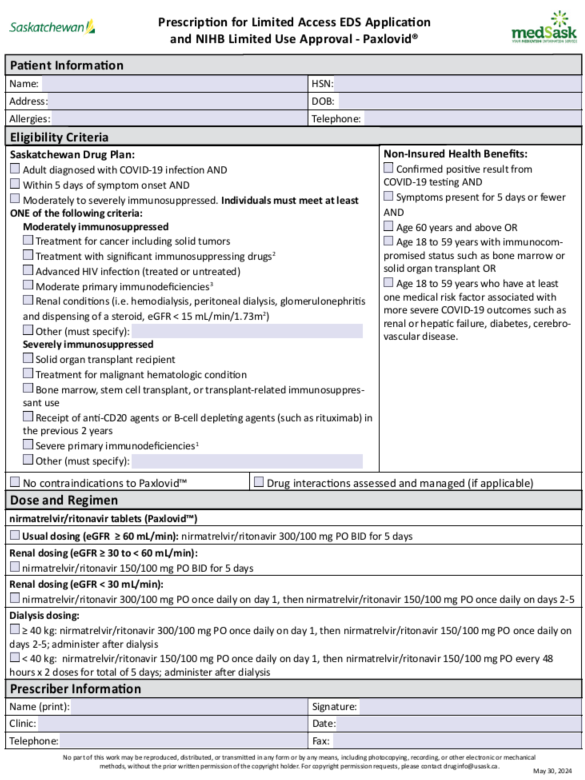
Key Points The landscape of COVID-19 has significantly changed since the pandemic.1,2 This is due in part to immunization, exposure from infection, and emergence of less virulent variants.2 Most people, especially those with immunity, will have mild, self-limited COVID-19 disease.2 Antivirals, like Paxlovid™, prevent hospitalization and death in people at high risk for progressing to severe COVID-19.1,3,4 Paxlovid™ has not been shown to help with symptom duration and severity.5 Evidence shows that moderately to severely immunosuppressed people are at the highest risk and are most likely to benefit from antivirals like Paxlovid™.1,4 Access to AntiviralsCOVID-19 TestingSaskatchewan.ca Testing and Treatment Information7 Rapid antigen self-testing: Under the Saskatchewan Drug Plan COVID-19 Rapid Antigen Test Assessment and Distribution Program, RATs are provided at no cost to high risk individuals and available at community pharmacies. RATs are available at no cost only to Saskatchewan residents who are at high risk for severe outcomes as follows: Meets the Drug Plan and Extended Benefits Branch’s (DPEBB’s) Exception Drug Status (EDS) criteria for Paxlovid™; OR have coverage for Paxlovid™ though Non-Insured Health Benefits Branch (NIHB). See Paxlovid™ Prescribing and Eligibility Criteria below. Individuals can be directed to a community pharmacy to request a RAT and the pharmacist will assess the individual for eligibility. The medSask COVID-19 Testing Assessment for Community Pharmacies form is used to determine which individuals are eligible to receive a RAT kit. If eligible, the tests will be provided free of charge. Note that while all community pharmacies may participate in the program, some may not have tests in stock, so it may be advisable to confirm test availability. For individuals not covered by the provincial testing program, RATs may be purchased at community pharmacies and other local retailers. PCR testing: Can be ordered by physicians and nurse practitioners. Other links: Access to COVID-19 Antivirals – Remdesivir & Paxlovid™ Saskatchewan.ca COVID-19 Treatments10Veklury® (remdesivir)Healthline 811 is not accepting referrals for COVID-19 antiviral therapy. Transplant and Oncology patients need to be referred to their specialist team. Some specialist prescribers/programs may have capacity to order and administer within Saskatchewan Health Authority facilities. Planning is underway to update the provincial order set and establish a pathway for access to remdesivir. Paxlovid™ (nirmatrelvir/ritonavir)For Saskatchewan Drug Plan coverage, pharmacies that receive a prescription will submit the EDS request using the online Limited Access Exception Drug Status Application. Approval will be in real-time. Only participating pharmacies will be able to submit an online EDS claim. See the Pharmacies Dispensing Paxlovid™ in Saskatchewan interactive map.11 Paxlovid™ Prescribing and Eligibility CriteriaPatients not eligible for Paxlovid™ should be reminded that Paxlovid™ only benefits people at high risk for hospitalization or death from COVID-19 infection. Paxlovid™ is not necessary for most people who are not high risk. Patients who do not need Paxlovid™ should be counselled on how to manage their symptoms at home and reminded to take measures to prevent the spread of their illness. Eligibility CriteriaNote that only Saskatchewan Drug Plan and NIHB criteria are listed here. Third-party payors often follow provincial eligibility criteria, but coverage and eligibility may vary among plans. Prescribers are advised to discuss cost and coverage with patients when prescribing. Saskatchewan Drug Plan Eligibility Criteria for Paxlovid™The eligibility criteria for Paxlovid™ have been updated to include only those individuals who are moderately to severely immunocompromised. The EDS criteria aligns with current evidence and Canada's Drug Agency recommendation.1 EDS criteria for Paxlovid™For the treatment of moderately or severely immunosuppressed adult patients diagnosed with COVID-19 infection. Paxlovid™ MUST be initiated within 5 days of symptom onset. Prescribers need to indicate the specific condition that confers immunosuppression. Examples of moderate immunosuppression may include: Treatment for cancer including solid tumors Treatment with significant immunosuppressing drugsb Advanced HIV infection (treated or untreated) Moderate primary immunodeficienciesc Renal conditions (i.e. hemodialysis, peritoneal dialysis, glomerulonephritis and dispensing of a steroid, eGFR < 15 mL/min/1.73m2) Other (to be specified by the prescriber) Examples of severe immunosuppression may include: Solid organ transplant recipients Treatment for malignant hematologic conditions Bone marrow, stem cell transplant, or transplant-related immunosuppressant use Receipt of anti-CD20 agents or B-cell depleting agents (such as rituximab) in the previous 2 years Severe primary immunodeficienciesa Other (to be specified by the prescriber) a - Severe immunodeficiencies include combined immunodeficiencies affecting T cells, immune dysregulation (particularly familial hemophagocytic lymphohistiocytosis), or type 1 interferon defects (caused by a genetic primary immunodeficiency disorder or secondary to anti-interferon autoantibodies). b - Immunosuppressing drugs such as a biologic in the past 3 months, oral immune-suppressing medication in the past months, oral steroid (20 mg/day of prednisone equivalent on an ongoing basis) in the past month, or immune-suppressing infusion or injection in the past 3 months. c - Includes a primary immunodeficiency13 with a genetic cause at any time or a primary immunodeficiency due to immunoglobulin replacement therapy in the past year. Non-Insured Health Benefits (NIHB) Eligibility Criteria for Paxlovid™Paxlovid™ is an open benefit. No prior approval is required. The NIHB drug benefit list can be accessed here.14 Paxlovid™ DistributionInformation about Paxlovid™ Distribution, Prescribing, and Assessment for pharmacies can be found on the Saskatchewan Drug Plan webpage.15 Clinical Information Pharmacist prescribers: The prescribing guideline for Mild COVID-19 is available under the "Pharmacist Prescribing" tab. Prescribing ResourcesGeneral Information about COVID-19General Information about COVID-19 Saskatchewan and Canada Epidemiology Data COVID-19 Signs and Symptoms 22 Post-COVID/Long COVID Treatment of Mild-Moderate COVID-19The following are general links for information about treating mild-moderate COVID-19. Consult local resources when considering the use of antivirals as eligibility criteria varies (see Paxlovid™ Prescribing and Eligibility Criteria above). Paxlovid™Indications Product Monograph29 Patients who do not need Paxlovid™ should be counselled on how to manage their symptoms at home and reminded to take measures to prevent the spread of their illness. Contraindications and Cautions Product Monograph29ContraindicationsHypersensitivity reaction - a history of clinically significant hypersensitivity reactions (e.g., toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome) to its active ingredients (nirmatrelvir or ritonavir) or any other components of the product.29 Drug interactions - concomitant use of drugs that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions or drugs that are potent CYP3A inducers where significantly reduced nirmatrelvir/ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance.29 See Drug Interactions. CautionsRenal impairment – individuals with renal impairment need dose adjustment as follows:29,35,36 eGFRDose60 to < 90 mL/min no adjustment required nirmatrelvir 300 mg with ritonavir 100 mg twice daily for 5 days ≥ 30 to < 60 mL/min nirmatrelvir 150 mg with ritonavir 100 mg twice daily for 5 days < 30 mL/min^ nirmatrelvir 300 mg with ritonavir 100 mg once on day 1, then nirmatrelvir 150 mg with ritonavir 100 mg once daily on days 2-5 Dialysis^ Intermittent hemodialysis and peritoneal dialysis: nirmatrelvir 300 mg with ritonavir 100 mg once on day 1, then nirmatrelvir 150 mg and ritonavir 100 mg once daily on days 2-5; administer after hemodialysis ^not recommended per product monograph.29 Dosing per clinical references (evidence from post-marketing). Pre-existing liver disease, liver enzyme abnormalities, hepatitis - hepatic transaminase elevations, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir.29 HIV drug resistance – use of Paxlovid™ may lead to a risk of HIV developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection.29 Drug interactions – concomitant use of medications metabolized by CYP3A or medications that inhibit or induce CYP3A must be reviewed with therapy not provided to patients with contraindicated interactions and other interactions appropriately managed.29 See Drug Interactions. Special populationsPediatrics: safety and efficacy have not been established in patients < 18 years of age. Saskatchewan Drug Plan coverage EDS criteria only includes adults as eligible for treatment with Paxlovid™. Not indicated for use in pediatrics per Canadian monograph29 however, the usual adult dose has been used in pediatric patients 12 years of age and older and weighing at least 40 kg with COVID-19 disease who are at high risk for progression to severe COVID-19.35,37,38 Pregnancy: limited data in pregnant people. Monograph suggests Paxlovid™ should be used only if the potential benefits outweigh the potential risk.29 Expert consensus (BCCDC, Ontario, ACOG) suggests that the use may be considered.24,33,39 Lactation: limited data in lactating people. Monograph suggests breast/chest feeding should be discontinued during treatment and for 48 hours after completing treatment.29 Expert consensus24,33 (BCCDC, Ontario) suggests that use may be considered. See LactMed for additional information.40 Drug InteractionsBoth nirmatrelvir and ritonavir have several drug-drug interactions, some of which will require management and others which contraindicate the use of Paxlovid™.29 Refer transplant patients and patients undergoing active cancer treatment to their specialist team for Paxlovid™ prescribing to ensure drug interactions with co-administered medications are managed appropriately.29,41,42 A comprehensive evaluation of drug interactions (including prescription, non-prescription, and recreational drugs, and natural health products) is required when considering the use of Paxlovid™. Prescribers must manage drug interactions and communicate the management strategy to patients. Prescribers or pharmacists may need to follow up to ensure temporarily adjusted medications are correctly restarted. Comprehensive drug interaction checker: University of Liverpool - COVID-19 Drug Interactions42 Other resources to check and manage drug interactions: Managing Drug InteractionsRitonavir is a CYP 3A4 inhibitor29 and may decrease the metabolism of medications dependent on CYP 3A. In those medications that require CYP 3A4 for clearance, elevated concentrations may occur. This could result in a serious or life-threatening reaction. In those medications that require CYP 3A4 for activation, such as prodrugs, reduced concentrations may occur. This could result in a decreased therapeutic effect of the medication. Nirmatrelvir and ritonavir are both major CYP 3A substrates:29 CYP 3A inducers may increase metabolism of Paxlovid™ which results in potential for loss of virologic response and/or development of resistance. Potential management strategies include:41,42 Adjusting the dose of interacting medication Temporarily holding the interacting medication Stopping the interacting medication Using an alternative COVID-19 therapeutic (i.e.: remdesivir) or an alternative to the interacting medication Continuing the interacting medication with monitoring Note that inhibition of CYP3A due to a Paxlovid™ interaction lasts about 2 days after stopping Paxlovid™.41 Dose adjustments, holding the interacting medication, or use of an alternative should occur for the entire course of Paxlovid™, plus 2 additional days after stopping, for a total of 7 days.41 Remember: a drug interaction in the table deemed “manageable” does not necessarily mean it is manageable for every patient. Consider additional patient factors that affect their ability to properly manage the drug interaction, such as: Do they understand which medication to hold or to split in half? Are they able to split the tablet themselves? Do they receive compliance packs which require manipulating? Do they comprehend when to restart their medication? Notable/common drug interactions (not exhaustive): Special attention is warranted when co-administering Paxlovid™ with medications that have a narrow therapeutic window:29,41 Anticoagulants (apixaban, rivaroxaban, warfarin) Anticonvulsants (carbamazepine, oxcarbazepine, phenytoin, phenobarbital, primidone) Antiarrhythmics (amiodarone, disopyramide, dronedarone, flecainide, propafenone, quinidine) Digoxin Opioids (particularly fentanyl) Medications that reduce levels of Paxlovid™:41 Rifampin St John’s Wort Interactions where stopping or switching may not be appropriate:41 Bosentan, sildenafil, tadalafil, vardenafil (for pulmonary hypertension) Clopidogrel, ticagrelor Clozapine Eplerenone Lurasidone, pimozide, quetiapine Combination oral contraceptives (patients are encouraged to use additional protection while using Paxlovid™ because levels of estrogen are reduced) Certain benzodiazepines Salmeterol Felodipine, nifedipine Ivabradine Others41 Some statins PDE4 inhibitors for erectile dysfunction Domperidone Dihydroergotamine Additional Clinical InformationWithin 5 days and for 5 days: Paxlovid™ is recommended within 5 days of symptom onset. Data from EPIC HR comparing earlier (<3 days) to later (3–5 days) treatment showed no difference in efficacy.2 Studies looking at people treated with Paxlovid™ beyond 5 days of symptom onset showed Paxlovid™ only works well when given within 5 days of symptom onset.45,46 Paxlovid™ is used for a total of 5 days. The benefit of extended courses or additional courses is uncertain.2 Paxlovid™ rebound: some people report a return of symptoms and test positivity a few days after completing a course of Paxlovid™. This may occur whether or not Paxlovid™ has been used.35,46 Recurrent symptoms are generally mild.46 Retreatment with Paxlovid™ is not routinely recommended, especially if symptoms are improving, but may be considered in people with significant immunosuppression.46 Side effects: most commonly reported side effects are dysgeusia (5%) and diarrhea (3%).29 Other side effects include nausea, vomiting, headache, malaise, and hypertension.29,35 Pharmacy Information - RAT Distribution
For information about access to testing, see COVID-19 Testing above. Rapid antigen tests (RATs) for self-testing are being provided by the Ministry of Health at no cost to individuals at high risk for severe outcomes (hospitalization, death) from COVID-19. Under the COVID-19 Rapid Antigen Tests Assessment and Distribution Program, RATs are to be distributed to individuals who may be eligible for COVID-19 antiviral treatment according to Saskatchewan Health EDS criteria or through Non-Insured Health Benefits (NIHB). The medSask COVID-19 Testing Assessment for Community Pharmacies form is used to determine which individuals are eligible to receive a RAT kit. Refer to DPEBB COVID-19 Rapid Antigen Tests Assessment and Distribution Program Policy15 for details. Contact UsmedSask for Clinical QuestionsFor questions about the clinical use of Paxlovid™, contact medSask. Email: druginfo@usask.ca Phone Numbers: 1-800-667-3425 (Saskatchewan) 306-966-6340 (Saskatoon) Drug Plan and Extended Benefits Branch (DPEBB) for Eligibility or Pharmacy Billing QuestionsFor questions about Saskatchewan Drug Plan eligibility or pharmacy billing, contact DPEBB. Email: DPEBimmunizations@health.gov.sk.ca Phone Numbers: 1-800-667-7581 (Saskatchewan) 306-787-3317 (Regina) Pharmacies interested in dispensing Paxlovid™ can register with DPEBB by emailing DPEBimmunizations@health.gov.sk.ca . ReferencesReferences Canadian Journal of Health Technologies. CADTH Reimbursement Recommendation Nirmatrelvir-Ritonavir (Paxlovid). 2024;4(4):1-23. Accessed May 29, 2024. https://www.cadth.ca/sites/default/files/DRR/2024/SR0808%20Paxlovid%20-%20Final%20Rec.pdf Grant JM, Lam J, Goyal SV, et al. AMMI Canada Practice Point: Updated recommendations for treatment of adults with symptomatic COVID-19 in 2023-2024. J Assoc Med Microbiol Infect Dis Can. 2024;8(4):245-252. Published 2024 Jan 16. doi:10.3138/jammi-2023-12-07. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10797770/ Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386(15):1397-1408. doi:10.1056/NEJMoa2118542. https://pubmed.ncbi.nlm.nih.gov/35172054/ Dormuth CR, Kim JD, Fisher A, Piszczek J, Kuo IF. Nirmatrelvir-Ritonavir and COVID-19 Mortality and Hospitalization Among Patients With Vulnerability to COVID-19 Complications [published correction appears in JAMA Netw Open. 2024 Feb 5;7(2):e241976]. JAMA Netw Open. 2023;6(10):e2336678. Published 2023 Oct 2. doi:10.1001/jamanetworkopen.2023.36678. https://pubmed.ncbi.nlm.nih.gov/37782496/ Hammond J, Fountaine RJ, Yunis C, et al. Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19. New England Journal of Medicine. 2024 Apr 4;390(13):1186-95. DOI: 10.1056/NEJMoa2309003 Government of Saskatchewan. COVID-19. Testing and Treatment Information. Accessed September 10, 2025. https://www.saskatchewan.ca/government/health-care-administration-and-provider-resources/treatment-procedures-and-guidelines/public-health-issues/respiratory-illnesses/covid-19/testing-information Paxlovid. Product Monograph. Pfizer Canada ULC. Updated January 17, 2022. Accessed May 28, 2024. https://pdf.hres.ca/dpd_pm/00073991. Government of Canada. Testing for COVID-19: When to get tested and testing results. Updated August 28, 2025. Accessed September 10, 2025. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/testing/diagnosing.html Government of Canada. Information for patients: A guide to antigen self-testing for COVID-19. Updated February 16, 2023. Accessed September 10, 2025. https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/testing-screening-contact-tracing/information-patients-guide-self-testing.html Government of Saskatchewan. COVID-19 Treatments. Accessed September 10, 2025. https://www.saskatchewan.ca/government/health-care-administration-and-provider-resources/treatment-procedures-and-guidelines/public-health-issues/respiratory-illnesses/covid-19/testing-information/covid-19-treatment Government of Saskatchewan. Pharmacies Prescribing and Dispensing Paxlovid in Saskatchewan. Accessed September 10, 2025. https://www.saskatchewan.ca/government/health-care-administration-and-provider-resources/treatment-procedures-and-guidelines/public-health-issues/respiratory-illnesses/covid-19/testing-information/covid-19-treatments/pharmacies-with-paxlovid Therapeutics Initiative. How Useful is Paxlovid in 2024? Therapeutics Letter 149, April 18, 2024. Accessed September 10, 2025. https://www.ti.ubc.ca/2024/04/18/how-useful-is-paxlovid-in-2024/ Immune Deficiency Foundation. Types of PI. Accessed September 10, 2025. https://primaryimmune.org/understanding-primary-immunodeficiency/types-of-pi Express Scripts. Drug Benefit List. https://nihb-ssna.express-scripts.ca/en/0205140506092019/16/160407 Saskatchewan Drug Plan. Pharmacy Professional Services, COVID-19 Programs. Accessed September 10, 2025. https://formulary.drugplan.ehealthsask.ca/COVIDImmunizationProgram Government of Canada. COVID-19: For health professionals. Updated February 28, 2025. Accessed September 10, 2025. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals.html World Health Organization. Coronavirus disease (COVID-19). Accessed September 10, 2025. Government of Canada. Coronavirus disease (COVID-19). Updated August 27, 2025. Accessed September 10, 2025. https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19.html Government of Saskatchewan. COVID-19 Situation Reports (CRISP). Accessed September 10, 2025. https://www.saskatchewan.ca/government/health-care-administration-and-provider-resources/treatment-procedures-and-guidelines/public-health-issues/respiratory-illnesses/covid-19/cases-and-risk-of-covid-19-in-saskatchewan Government of Canada – Canadian Respiratory Virus Surveillance Report. Accessed September 10, 2025. https://health-infobase.canada.ca/respiratory-virus-surveillance/ Government of Canada. Wastewater monitoring dashboard. Accessed September 10, 2025. https://health-infobase.canada.ca/wastewater/ Government of Canada. COVID-19 signs, symptoms and severity of disease: A clinician guide. Updated June 1, 2022. Accessed September 10, 2025. Government of Canada. Post COVID-19 Condition (long COVID). Updated October 10, 2024. Accessed September 10, 2025. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/post-covid-19-condition.html BC COVID Therapeutics Committee. Clinical Practice Guide for the Use of Therapeutics in Mild-Moderate COVID-19. Updated April 2025. Accessed September 10, 2025. https://www.bccdc.ca/Health-Professionals-Site/Documents/COVID-treatment/ClinicalPracticeGuide_Therapeutics_MildModerateCOVID.pdf Ontario Health. Recommendations for Antiviral Therapy for Adults with Mild to Moderate COVID-19. Updated April 2, 2024. Accessed September 10, 2025. https://www.ontariohealth.ca/content/dam/ontariohealth/documents/recommendations-for-antiviral-therapy-for-adults-with-mild-to-moderate-covid-19.pdf Crawley A, LeBras M, Regier L. COVID-19 COVID-19- Vaccines, Prevention and Therapies for Outpatients. Updated September 2025. Accessed September 10, 2025. https://www.rxfiles.ca/RxFiles/uploads/documents/cht-COVID-19.pdf Agarwal A, Hunt B J, Stegemann M, Rochwerg B, Lamontagne F, Siemieniuk R A et al. A living WHO guideline on drugs for covid-19 BMJ 2020; 370 :m3379 doi:10.1136/bmj.m3379 IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Updated June 4, 2025. Accessed September 10, 2025. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ Paxlovid. Product Monograph. Pfizer Canada ULC. Updated August 1, 2025. Accessed September 10, 2025. https://pdf.hres.ca/dpd_pm/00081268.PDF Xie Y,1 Choi T, Al-Aly Z. Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern Med. 2023 Jun 1;183(6):554-564. doi: 10.1001/jamainternmed.2023.0743. PMID: 36951829; PMCID: PMC10037200. Geng LN, Bonilla H, Hedlin H, Jacobson KB, Tian L, Jagannathan P, Yang PC, Subramanian AK, Liang JW, Shen S, Deng Y, Shaw BJ, Botzheim B, Desai M, Pathak D, Jazayeri Y, Thai D, O'Donnell A, Mohaptra S, Leang Z, Reynolds GZM, Brooks EF, Bhatt AS, Shafer RW, Miglis MG, Quach T, Tiwari A, Banerjee A, Lopez RN, De Jesus M, Charnas LR, Utz PJ, Singh U. Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial. JAMA Intern Med. 2024 Sep 1;184(9):1024-1034. doi: 10.1001/jamainternmed.2024.2007. Erratum in: JAMA Intern Med. 2024 Sep 1;184(9):1137. doi: 10.1001/jamainternmed.2024.3735. PMID: 38848477; PMCID: PMC11161857. Sawano M, Bhattacharjee B, Caraballo C, Khera R, Li SX, Herrin J, Christian D, Coppi A, Warner F, Holub J, Henriquez Y, Johnson MA, Goddard TB, Rocco E, Hummel AC, Mouslmani MA, Hooper WB, Putrino DF, Carr KD, Charnas L, De Jesus M, Nepert D, Abreu P, Ziegler FW 3rd, Spertus JA, Iwasaki A, Krumholz HM. Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): a double-blind, randomised, placebo-controlled, phase 2, decentralised trial. Lancet Infect Dis. 2025 Aug;25(8):936-946. doi: 10.1016/S1473-3099(25)00073-8. Epub 2025 Apr 3. PMID: 40188838. Ontario Health. Frequently asked questions on antiviral therapy for adults with mild to moderate COVID-19. Updated September 3 2024. Accessed September 10, 2025. https://www.ontariohealth.ca/content/dam/ontariohealth/documents/faqs-on-antiviral-therapy-for-adults-with-mild-to-moderate-covid-19.pdf Reis S, Metzendorf MI, Kuehn R, Popp M, Gagyor I, Kranke P, Meybohm P, Skoetz N, Weibel S. Nirmatrelvir combined with ritonavir for preventing and treating COVID-19. Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD015395. doi: 10.1002/14651858.CD015395.pub3. PMID: 38032024; PMCID: PMC10688265. Nirmatrelvir and Ritonavir. UpToDate Lexidrug. Wolters Kluwer. Updated September 10, 2025. Accessed September 10, 2025. https://online-lexi-com/ Cho WJ, Harden D, Moreno D, et al. Oral antiviral therapies for COVID-19 in patients with advanced chronic kidney disease or kidney failure. Nephrol Dial Transplant. 2023;38(8):1912-1914. doi:10.1093/ndt/gfad058 Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid™. Updated March 2024. Accessed May 28, 2024. https://www.fda.gov/media/155050/download Wong CKH, Lau KTK, Au ICH, Chan SHS, Lau EHY, Cowling BJ, Leung GM. Effectiveness of nirmatrelvir/ritonavir in children and adolescents aged 12-17 years following SARS-CoV-2 Omicron infection: A target trial emulation. Nat Commun. 2024 Jun 8;15(1):4917. doi: 10.1038/s41467-024-49235-8. PMID: 38851796; PMCID: PMC11162460. American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetricians-gynecologists, obstetrics. Updated August 2025. Accessed September 10, 2025. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics National Library of Medicine. Drugs and Lactation Database (LactMed®). Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Accessed September 10, 2025. https://www.ncbi.nlm.nih.gov/books/NBK501922/. BC COVID Therapeutics Committee. Practice Tool – Drug-Drug Interactions and Contraindications. Updated May 22, 2024. Accessed September 10, 2025. https://www.bccdc.ca/Health-Professionals-Site/Documents/COVID-treatment/PracticeTool3_DrugInteractionsContraindications.pdf University of Liverpool. Covid-19 Drug Interactions; Interaction checker. c20245. Accessed September 10, 2025. https://www.covid19-druginteractions.org/checker Infectious Diseases Society of America. Management of Drug Interactions With Nirmatrelvir/Ritonavir: Resource for Clinicians. Updated May 6, 2022. Accessed September 10, 2025. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/management-of-drug-interactions-with-nirmatrelvirritonavir-paxlovid/ University Health Network. Paxlovid Prescribing & Drug Interaction Information. Accessed September 10, 2025. https://hivclinic.ca/paxlovid-prescribing-drug-interaction-information/ Wang Y, Zhao D, Liu X, Chen X, Xiao W, Feng L. Early administration of Paxlovid reduces the viral elimination time in patients infected with SARS-CoV-2 Omicron variants. J Med Virol. 2023 Jan;95(1):e28443. doi: 10.1002/jmv.28443. PMID: 36579782; PMCID: PMC9880690.) Cohen P, Gebo K. COVID-19: Management of adults with acute illness in the outpatient setting. UpToDate. Wolters Kluwer. Updated June 12,2025. Cited September 10,2025. https://www.uptodate.com/contents/covid-19-management-of-adults-with-acute-illness-in-the-outpatient-setting No part of this work may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the copyright holder. For copyright permission requests, please contact druginfo@usask.ca. Posted: May 30, 2024 Last update: September 2025 (责任编辑:) |